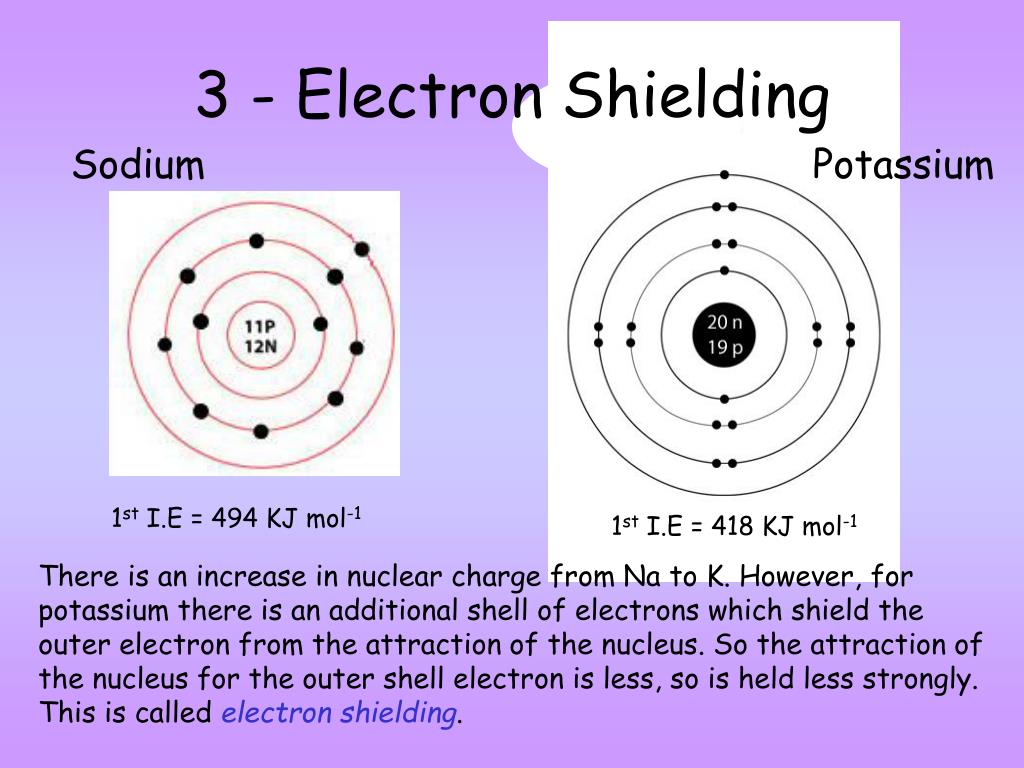
How Does Electron Shielding Change Down A Group . In general, ionization energies increase from left to right and decrease down a group; Electrons in an s s orbital can shield p p. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Shielding increases down a group because the nuclear core is farther removed from the valence electrons. In general, ionization energy increases across a period and decreases down a group. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more. Across a period, efective nuclear charge increases as. However, there are variations in these trends that would.
from www.slideserve.com
However, there are variations in these trends that would. In general, ionization energy increases across a period and decreases down a group. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Shielding increases down a group because the nuclear core is farther removed from the valence electrons. In general, ionization energies increase from left to right and decrease down a group; The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more. Across a period, efective nuclear charge increases as. Electrons in an s s orbital can shield p p.
PPT Lesson objectives • Define first ionisation energy and successive
How Does Electron Shielding Change Down A Group However, there are variations in these trends that would. Across a period, efective nuclear charge increases as. Electrons in an s s orbital can shield p p. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In general, ionization energies increase from left to right and decrease down a group; In general, ionization energy increases across a period and decreases down a group. Shielding increases down a group because the nuclear core is farther removed from the valence electrons. However, there are variations in these trends that would. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 How Does Electron Shielding Change Down A Group The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In general, ionization energy increases across a period and decreases down a group. Electrons in an s s orbital can. How Does Electron Shielding Change Down A Group.
From www.slideshare.net
Periodic trends How Does Electron Shielding Change Down A Group Shielding increases down a group because the nuclear core is farther removed from the valence electrons. However, there are variations in these trends that would. Across a period, efective nuclear charge increases as. Electrons in an s s orbital can shield p p. In general, ionization energy increases across a period and decreases down a group. In chemistry, the shielding. How Does Electron Shielding Change Down A Group.
From www.teachoo.com
[Term 2] Account for (b) In case of transition elements, ions of same How Does Electron Shielding Change Down A Group The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. However, there are variations in these trends that would. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Shielding increases down a group because the nuclear core is farther. How Does Electron Shielding Change Down A Group.
From www.chemistrystudent.com
Group 7 Halogens and their Electronegativities (ALevel) ChemistryStudent How Does Electron Shielding Change Down A Group In general, ionization energies increase from left to right and decrease down a group; However, there are variations in these trends that would. In general, ionization energy increases across a period and decreases down a group. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in. How Does Electron Shielding Change Down A Group.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The How Does Electron Shielding Change Down A Group In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more. Electrons in an s s orbital can shield p p. However, there are variations in these trends that would. In general, ionization energies increase from left to right and decrease down a. How Does Electron Shielding Change Down A Group.
From byjus.com
What is shielding and deshielding in NMR? Give an example? How Does Electron Shielding Change Down A Group The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In general, ionization energy increases across a period and decreases down a group. Shielding increases down a group because the nuclear core is farther removed from the valence electrons. Across a period, efective nuclear charge increases as. The concept of. How Does Electron Shielding Change Down A Group.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID5583827 How Does Electron Shielding Change Down A Group The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Electrons in an s s orbital can shield p p. Shielding increases down a group because the nuclear core is farther removed from the valence electrons. In general, ionization energy increases across a period and decreases down a group. Across. How Does Electron Shielding Change Down A Group.
From thechemistrynotes.com
Shielding effect How Does Electron Shielding Change Down A Group Across a period, efective nuclear charge increases as. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In general, ionization energies increase from left to right and decrease down. How Does Electron Shielding Change Down A Group.
From www.chemistrysteps.com
NMR Chemical Shift ppm, Upfield, Downfield Chemistry Steps How Does Electron Shielding Change Down A Group However, there are variations in these trends that would. Across a period, efective nuclear charge increases as. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Shielding increases down a group because the nuclear core is farther removed from the valence electrons. In general, ionization energies increase from left. How Does Electron Shielding Change Down A Group.
From www.slideserve.com
PPT Drill 6 11/11 & 11/12/13 PowerPoint Presentation, free download How Does Electron Shielding Change Down A Group In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In general, ionization energy increases across a period and decreases down a. How Does Electron Shielding Change Down A Group.
From www.shemmassianconsulting.com
Atoms and Periodic Trends for the MCAT Everything You Need to Know How Does Electron Shielding Change Down A Group Electrons in an s s orbital can shield p p. In general, ionization energies increase from left to right and decrease down a group; The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge. How Does Electron Shielding Change Down A Group.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e How Does Electron Shielding Change Down A Group In general, ionization energy increases across a period and decreases down a group. Shielding increases down a group because the nuclear core is farther removed from the valence electrons. In general, ionization energies increase from left to right and decrease down a group; The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced. How Does Electron Shielding Change Down A Group.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding How Does Electron Shielding Change Down A Group Shielding increases down a group because the nuclear core is farther removed from the valence electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In chemistry, the shielding. How Does Electron Shielding Change Down A Group.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free How Does Electron Shielding Change Down A Group Electrons in an s s orbital can shield p p. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more. The concept. How Does Electron Shielding Change Down A Group.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e How Does Electron Shielding Change Down A Group In general, ionization energies increase from left to right and decrease down a group; However, there are variations in these trends that would. In general, ionization energy increases across a period and decreases down a group. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in. How Does Electron Shielding Change Down A Group.
From www.youtube.com
Shielding Effect in the Periodic Table Chemistry YouTube How Does Electron Shielding Change Down A Group The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In general, ionization energies increase from left to right and decrease down a group; Electrons in an s s orbital can shield p p. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge. How Does Electron Shielding Change Down A Group.
From www.slideserve.com
PPT Drill 6 11/11 & 11/12/13 PowerPoint Presentation, free download How Does Electron Shielding Change Down A Group However, there are variations in these trends that would. In chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between an electron and the nucleus in any atom with more. In general, ionization energy increases across a period and decreases down a group. The concept of electron shielding, in which intervening electrons act. How Does Electron Shielding Change Down A Group.
From slidetodoc.com
Trends in the Periodic Table IDENTIFYING THE PATTERNS How Does Electron Shielding Change Down A Group The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. In general, ionization energy increases across a period and decreases down a group. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Across a period, efective nuclear charge increases. How Does Electron Shielding Change Down A Group.